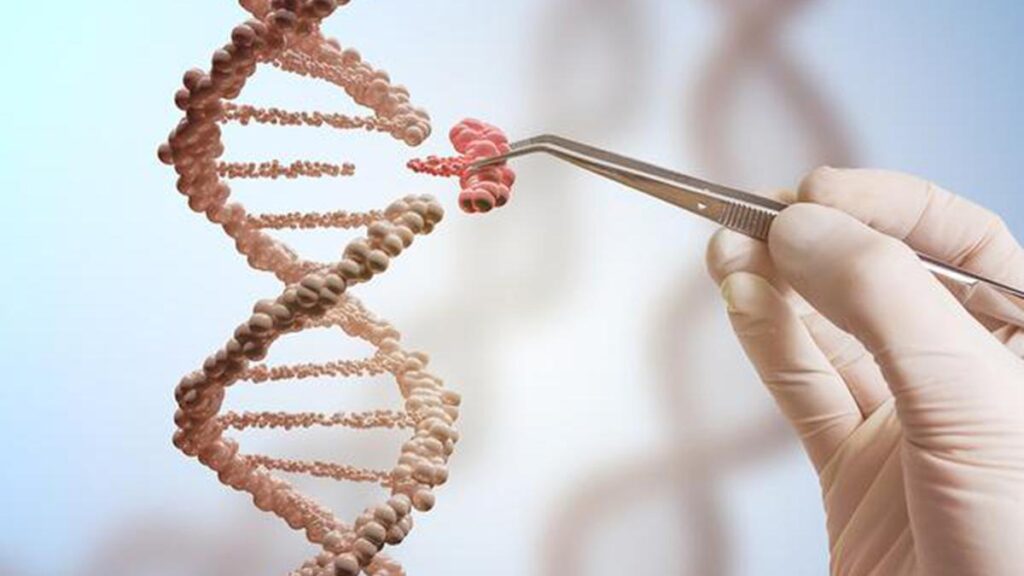
The fourth industrial revolution marked by the fusion of technologies is blurring the lines between the physical, digital and biological worlds. And it is at this intersection that the future of medicine is being written, with innovations like CRISPR and CAR-T therapy unlocking the potential to not only treat diseases but cure them. From editing the human genome to reprogramming immune cells to fight cancer, these approaches promise to transform healthcare as we know it. But as we stand on the brink of this new era, the question is no longer if these technologies will change lives — but how they will reshape the framework of human biology, ethics, and society itself.
In 2012, Jennifer Doudna and Emmanuelle Charpentier published their groundbreaking work, revealing that CRISPR/Cas9 could be re-engineered as a gene-editing tool. They won the Nobel Prize in Chemistry for this, in 2020.
On December 8, 2023, the United States’ Food and Drug Administration (FDA) approved of a gene therapy treatment for transfusion dependent beta-thalassemia and sickle cell anaemia (SCA) patients — a decision that will revolutionise medicine and change countless lives.
Yet, I find myself grappling with mixed emotions. While technology and medicine have always been intertwined, never have we imagined breathing life into extinct creatures or mending/tailoring our genes, ideas nothing short of science fiction.
What is CRISPR?
Casgevy and Lyfgenia, the two cell-based gene therapies approved by the FDA, utilise the CRISPR/Cas9 genome editing technology. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) /CRISPR-associated protein 9 (Cas 9) evolved naturally as a defence mechanism in bacteria and archaea. It was first reported in E. Coli in 1987 by Ishino et.al.
In a nutshell, the system serves as a genetic memory for past infections by incorporating a part of the viral genetic material into its own, so that the next time it is invaded, the bacteria is capable of recognising the virus and destroying it. The bacteria, in short, has developed an immunisation mechanism to ward off unwanted viral invaders.
Watch:2020 Chemistry Nobel for developing CRISPR/Cas9 genetic scissors
The CRISPR system is easy to manipulate. Researchers have adapted it as a tool to cut, delete, or add DNA sequences at precise locations opening doors to treating genetic disorders, in diagnostics, creating disease/drought resistant plants, or in de-extinction projects involving the woolly mammoth and the dodo.
The progress in CRISPR has been rapid. In only a decade since its discovery, we now have a technology with the potential to rewrite genetic code. As exciting as the prospect is, conversations around ethical, societal and safety issues must progress parallelly.
Ethical considerations
A siren rang across the scientific world in 2018, when Chinese scientist He Jiankui announced he had altered a gene in three human embryos to render resistance to HIV. He was imprisoned for three years in 2019 by a court in China. The scientific community also condemned his actions, and this self-regulation was crucial as it was a clear violation of guidelines that banned germline editing. But nonetheless “designer babies” became a reality. The sole purpose of Jiankui and his team experimenting with the embryos was to monitor the evolution of two babies, as the genetic intervention was different for each embryo. The idea of such tools in the hands of scientists like Jiankui is terrifying. In an article, Françoise Baylis, a bioethicist at Dalhousie University in Nova Scotia, rightly pointed out “There is a difference between making people better and making better people.”
There is also concern amongst the public that the wealthy will exploit this technology for genetic enhancement. While CRISPR, right now, is being used to treat monogenic disorders, it is capable of altering multiple genes. Admittedly however, factors like intelligence are complex traits influenced by many genes and the environment, making it difficult to tailor. In addition, CRISPR has also got its own safety problems, such as off-targeting, that aren’t fully understood yet.
While there is a consensus within the scientific community to use CRISPR for therapeutic purposes, incidents like the Chinese case are always possibilities.
And so, when benefits outweigh the risks where will scientists draw the line? Is it ethical to perform germline editing, even for the purpose of treating diseases? When it comes to human embryos, we face numerous unresolved moral questions and have much to learn about what is right and wrong in this rapidly evolving field.
Also Read:A reckless experiment: on gene edited babies
For many in the disabled community, their genetic anomalies are part of their identities. This raises important questions about the intersection of ableism and CRISPR, particularly regarding the potential implications of societal attitudes towards disability as genetic technologies become common. While most would choose gene therapies, there is a concern that such emerging technologies could reinforce the notion that disability is an abnormality rather than a natural aspect of human diversity. This makes it necessary to include disabled voices in discussions from the beginning, prior to clinical applications, and at the policy-making level.
The cost factor
Another factor is the exorbitant price of such technologies. Casgevy costs around $2.2 million. Sickle Cell Anaemia (SCA) is mostly seen in certain ethnic groups including people of African, Mediterranean and Middle Eastern descent. In India, SCA is relatively higher in tribal communities. Many people from these groups are economically disadvantaged and find it difficult to afford even primary healthcare. Indian researchers are working towards building a cost-effective treatment with CRISPR. But we are talking about a huge leap from basic – hydroxyurea – first line treatment in India to CRISPR, an advanced tool requiring skilled experts. With CRISPR in the market, it is of paramount importance to first initiate dialogues on health equity.
According to fundamental cause theory, health disparities persist because advancements in medicine tend to benefit the more advantaged segments of society, leaving disadvantaged groups with less access to new interventions. As seen during events like the COVID-19 pandemic, those with higher social status — who have more resources — can access healthcare more readily than minority populations, who often face barriers in obtaining similar benefits.
Will CRISPR help farmers, or will it help the agribusiness giants that invest in it? Will it find a way to reach the vulnerable or will it become a tool for the wealthy? And do we really need de-extinction projects when we are failing to protect extant animals? As we move into the future and CRISPR becomes easier to use and is better understood, how will future generations choose to alter human embryos on which currently there is a moratorium?
The questions are numerous and the answers blurry. It is still early to say when CRISPR will be affordable to a larger section of people. As these technologies emerge and evolve at a rapid speed, regulatory bodies must establish strict guidelines for ethical, societal, safety and environmental issues at the same pace.
CAR-T Cell Therapy
The same year that CRISPR’s use as a gene-editing tool was discovered, a six-year-old’s battle with acute lymphoblastic leukaemia (ALL) would define the entire field of CAR-T cell therapy. Chimeric Antigen Receptor (CAR) T cell therapy genetically alters a patient’s T cells (a type of white blood cell) to fight malignant tumour cells by targeting a protein on the surface of cancer cells.
When the experimental treatment was in its earliest days, a doctor, Stephen Grupp, was approached by Emily Whitehead’s parents looking for a miracle to save their daughter as other doctors had given up all hope. The brand-new therapy began its phase I clinical trial and Emily was enrolled. She became the first paediatric patient to receive the treatment and the first ever patient of any age to receive it for ALL.
Twelve years later, it’s clear the Whiteheads found the miracle they sought: Emily remains cancer-free. However, the journey was far from easy. Even doctors were uncertain of the treatment’s outcome, learning alongside Emily, as challenges arose throughout her care.
In 2017, the FDA approved the first CAR-T therapy, paving the way for hundreds of patients to receive the treatment, thanks in-part to courageous families like Emily’s. It is important to acknowledge though that not all outcomes are as happy as Emily’s. Some patients remain unresponsive or may relapse. Each case is unique and there is a learning curve with each patient.
How CAR-T therapy works
T cells possess receptors on their surface that can recognise antigens — proteins or molecules identified by the immune system — and when foreign antigens are detected, the immune system signals the T cells to destroy them.
However, cancer cells can sometimes express antigens that the body does not recognise as abnormal, preventing the immune system from sending T cells to attack them. In other instances, even if T cells are present, they may not be effective at eliminating cancer cells.
CAR-T cells are genetically engineered in a laboratory to include a new receptor that enables them to bind to and kill cancer cells. The development of CAR-T cells involves a multi-step process that begins with collecting T-cells from the patient through a procedure called leukapheresis. These T-cells are then modified in the lab to express CARs on their surface. The gene encoding the CAR is synthesised in the lab, and a vector — often a viral vector, is used to deliver the CAR gene into the T-cells.
Once re-engineered, the T-cells are multiplied in the lab to generate millions of cells, which are then sent back to the hospital for infusion into the patient. Typically, patients undergo chemotherapy before receiving the CAR-T cells. Different types of cancer have unique antigens, which means that each CAR-T cell therapy is designed to target a specific cancer antigen.

CAR-T cell therapy costs anywhere between ₹3 to 4 crore, excluding hospitalisation charges, rendering it unavailable to most people. While a few insurance plans do cover the expenses of the treatment in the United States, some procedures might not be covered. Logistics, travel and food are other expenses one needs to keep in mind while undergoing this treatment.
The unaffordability of CAR-T therapy fuelled Rahul Purwar’s vision of developing an indigenous version. Currently a professor at the Indian Institute of Bombay (IIT-B), Dr. Purwar returned to India in 2013 after completing his postdoctoral programme at Harvard Medical School. Along with his research students — Alka Dwivedi, Atharva Karulkar — and haemato-oncologists Gaurav Narula and Hasmukh Jain from Tata Memorial Hospital, it took a decade to bring this vision to fruition.
The process of designing CAR-T cells requires expertise. The researchers collaborated with experts at the National Cancer Institute (NCI) to overcome the challenges they faced. The team had to then approach the Central Drugs Standard Control Organisation (CDSCO) for clinical trial approval, which involved several rounds of revisions. It was also a first for CDSCO, as guidelines were not framed with respect to cell therapy, and they evolved along with the technology.
In October 2023, India got its first indigenous CAR-T therapy — NEXCAR19 — that costs around ₹45 lakh, a fraction of its U.S. counterpart. This sum, however, still remains unaffordable for a majority of Indians, for whom accessing even primary healthcare is difficult. Proximity to a well-equipped hospital is also necessary, as some cases may require intensive care utilisation after the treatment and follow-ups on a regular basis.

And so as with CRISPR, we circle back to the question of health equity with emerging medical technologies. Can we hope the prices will decrease once manufacturing increases? Can these technologies be implemented in sub-urban and rural India?
There is no doubt that CRISPR and CAR-T cell therapy are breakthroughs in the medical field. But because they are still in their initial stages, they come with a million-dollar price tag. Developing new therapies is expensive. In addition to the cost of research, manufacturing, labour, logistics, marketing, distribution and intellectual property development, comes the added work of regulatory approval.
But what does the future hold for such emerging trends that interweave different fields to pave the way for improving people’s lives, if the outcomes are but accessible to a handful?
(Soujanya Padikkal is a freelance content provider based in Hyderabad)
Published – February 24, 2025 11:41 am IST