Therapeutic proteins are increasingly becoming an indispensable part of healthcare, with their applications found in treating both infectious diseases, and non-communicable diseases such as diabetes and cancer.
What are they and how do they work?
Proteins are the largest class of biomolecules that drive cellular activities essential for life. Any aberration in their synthesis, processing or function, can lead to a state of disease. Therapeutic proteins are biological molecules that are largely derived from human proteins, whose pharmacological activity can span one of several mechanisms. These include the ability to inhibit or interfere with abnormal protein function, replace a deficient or dysfunctional protein, enhance existing cellular activity, introduce a novel function, or serve as a delivery vehicle for drugs.
This class of drug molecules commands certain clear advantage over small molecules (drugs of which make up around 90% of all pharmaceutical drugs at present) in several areas. Proteins have evolved to perform specific and very complex functions, and consequently, they are prone to cause fewer side effects. They are often highly potent and can circulate in the blood for a longer period, thus requiring shorter dosing frequencies.

The evolution of protein therapeutics
More than a century of research and development has shaped the field of protein therapeutics, bringing it to its current state. The first documented instance of a protein drug was serum therapy for diphtheria in the 1890s that saved thousands of lives and earned Emil Adolf von Behring the Nobel Prize for Medicine in 1901 for his contribution to this groundbreaking effort.
The extraction of insulin from animal sources to treat diabetes in the early part of the 20th century was another key milestone. Six decades later, in 1982, the first biosynthetic insulin produced by recombinant DNA technology was granted approval by the United States’ Food and Drug Administration (FDA) and heralded a new era in protein therapeutics.
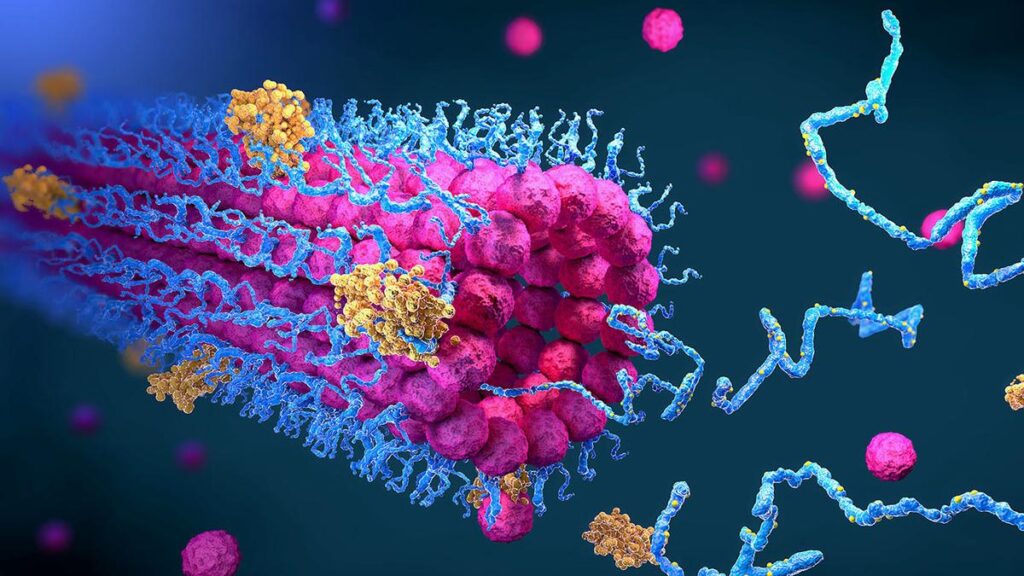
The discovery of monoclonal antibodies and the technology for their large-scale production ushered in a paradigm shift in drug discovery. This enabled far-reaching applications in diagnostics, biological research, targeted treatment of various ailments like cancer, immunologic diseases and infectious diseases. Rational engineering of proteins, to enhance their stability, specificity and potency along with techniques that allowed the reduction of immunogenic responses to the engineered proteins, expanded the therapeutic scope of this class of drug molecules.
The turn of the 21st century saw the advent of further technological advancements – in the testing of large numbers of compounds, in conjunction with the collection of synthesised proteins. These innovations led to the rapid discovery and development of fully human antibodies with better safety profiles and more desirable drug-like properties.

The current scenario
There are currently 894 therapeutic proteins approved for clinical use by the FDA. Due to their inherent nature, patent protection for protein-based drugs can be much stronger than for small molecules. Additionally, the FDA approval time for protein therapeutics has been faster than that for small molecules. A combination of these two considerations makes protein therapeutics very attractive from a financial perspective as well. A report by market research firm Spherical Insights valued the global therapeutic proteins market size at $341.1 billion in 2023 and has projected a growth that will see the market cross $600 billion by 2033.
Several emerging trends are redefining the scope and applications of protein drug discovery at present. Alternate protein scaffolds to antibodies, which dominate the therapeutic proteins field, are being explored for better targeting of specific diseases. Applications of Artificial Intelligence (AI) in drug discovery are accelerating the design and optimisation of protein therapeutics and making the processes more efficient.
Researchers are also developing novel therapeutic modalities, for example, by harnessing the body’s immune system to fight cancer as in CAR-T cell therapies, by engineering multi-specific and bi-specific antibodies to combat treatment-resistant cancers or autoimmune diseases with complex mechanisms. A need for biosimilars (biologics with highly similar properties to existing therapeutic molecules) and biobetters (biologics with improved properties), is also gaining traction, especially to increase global availability and affordability.

Combating existing challenges
Despite the exciting advancements being made, challenges, however, remain. For a protein therapeutic to be approved for clinical use, for instance, a significant challenge is the combination of a relatively low success rate of approval with long development timelines. This is reflected in the high cost of shepherding a therapeutic protein through the clinic to the pharmaceutical market.
Proteins are also prone to chemical and physical degradation, and this presents a challenge to the manufacturing processes in the drug development industry. By innovating methods to improve inefficiencies in each phase of the drug discovery and development programme, for example by employing AI, where applicable, to model and mitigate these deficiencies, some of these challenges can be overcome.
Another challenge is that protein drugs, when introduced into a human, can elicit an adverse immune response that can reduce or even eliminate the therapeutic effect of the biologic. This is an area that is ripe for innovation, where development of novel methods and models to accurately predict immunogenicity outcomes in a clinical trial, will help drastically reduce the cost and time of development.

Another limitation has to do with the repertoire of disease-causing proteins that can be targeted by protein-based therapeutics. Protein-based therapeutics have failed to target several highly relevant classes of disease-causing proteins, and this has to do with their inherent nature. It is well understood that proteins can fold into three-dimensional structures and these are the regions that are targeted by both small and large molecule drug discovery programmes. What is less appreciated, even in the scientific field, is the fact that proteins also possess domains that are very important biologically, but do not have a set structure. Targeting these intrinsically disordered regions (IDRs) of proteins for therapeutic intervention, in a programmable manner, is the next frontier for drug discovery campaigns.
While the challenges are many, recent advances in biotechnology, synthetic biology and application of breakthroughs in AI to protein engineering, offer hope to all the many patients suffering from debilitating conditions and diseases for which no therapies exist.
(Shankar Shastry is co-founder & head of protein sciences, Aikium Inc., Berkeley, California, USA)
Published – February 28, 2025 10:30 am IST